Research Topics:
Other Protein studies from our lab
Receptor Tyrosine Kinase Like Orphan Receptors (RORs) are poorly characterized receptor tyrosine kinases (RTKs) involved in embryonic development and muscle differentiation. The most relevant insights regarding the implications of RORs emerge from the evidence of ROR gene overexpression during cancer progression, in particular with the development of solid and hematopoietic malignancies. In particular, the strongly restricted ROR1 expression in cancer cells and its low-level of expression in healthy adult tissues, suggested that ROR1 could be an interesting marker for targeted cancer therapy. Parallel to drug discovery campaigns focusing on development of small molecules targeting the intracellular kinase domain of ROR2, the recent characterization of antibodies specifically targeting the KRDs of human ROR1 and ROR2 displaying selective antitumor activity in vitro and in vivo in mice has boosted the interest towards these orphan receptors, and in particular towards their ectodomain architecture. In this context, we carried out recombinant expression, crystallization, and structure determination of the isolated KRD of human ROR1 (hROR1-KRD) at high resolution.
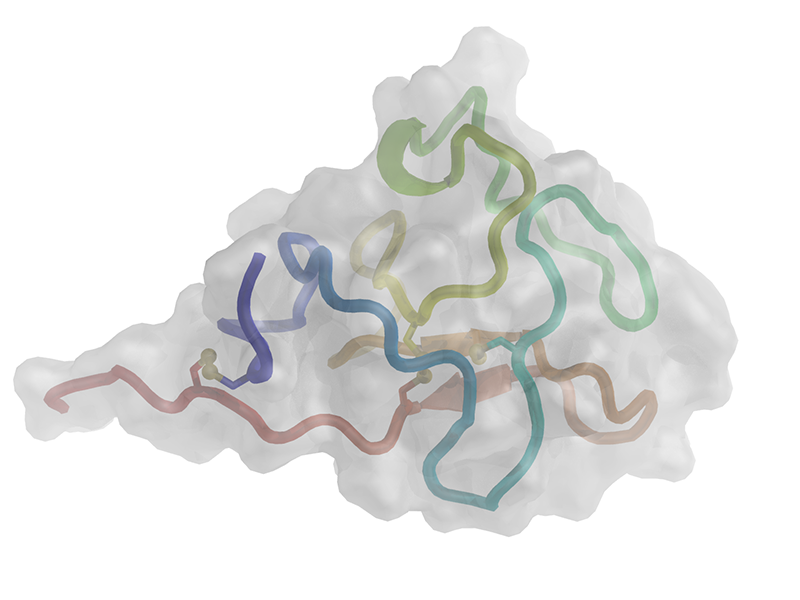
|
Cartoon representation of the hROR1-KRD. The polypeptide chain is colored from the N-terminus (blue) to the C-terminus (red). Disulfide bonds are shown as ball-and-sticks, and residues constituting the boundaries of the few secondary structure elements are labeled. |
PDB Files:
Raw Data in Repository:
Publications:
Crystal structure of the kringle domain of human receptor tyrosine kinase-like orphan receptor 1 (hROR1).
Acta Crystallographica section F - Structural Biology Communications, F78, 185-192. (2022)
PubMed
Thanks to a collaboration with Dr. Alessandra Villa (Philochem AG) and Prof. Dario Neri (ETH Zurich), we have been involved in structural characterization of clinical-grade immunocytokines. We have determined the three-dimensional structure of the common L19 diabody fragment and used SEC-SAXS and negative stain EM to characterize three full-length flexible immunocytokines. The results obtained indicate that low-resolution molecular structure characterizations can provide useful complementary insights for the quality control of immunocytokines, constituting a powerful tool to guide the design and the subsequent optimization steps towards clinical enhancement of these chimeric protein reagents.
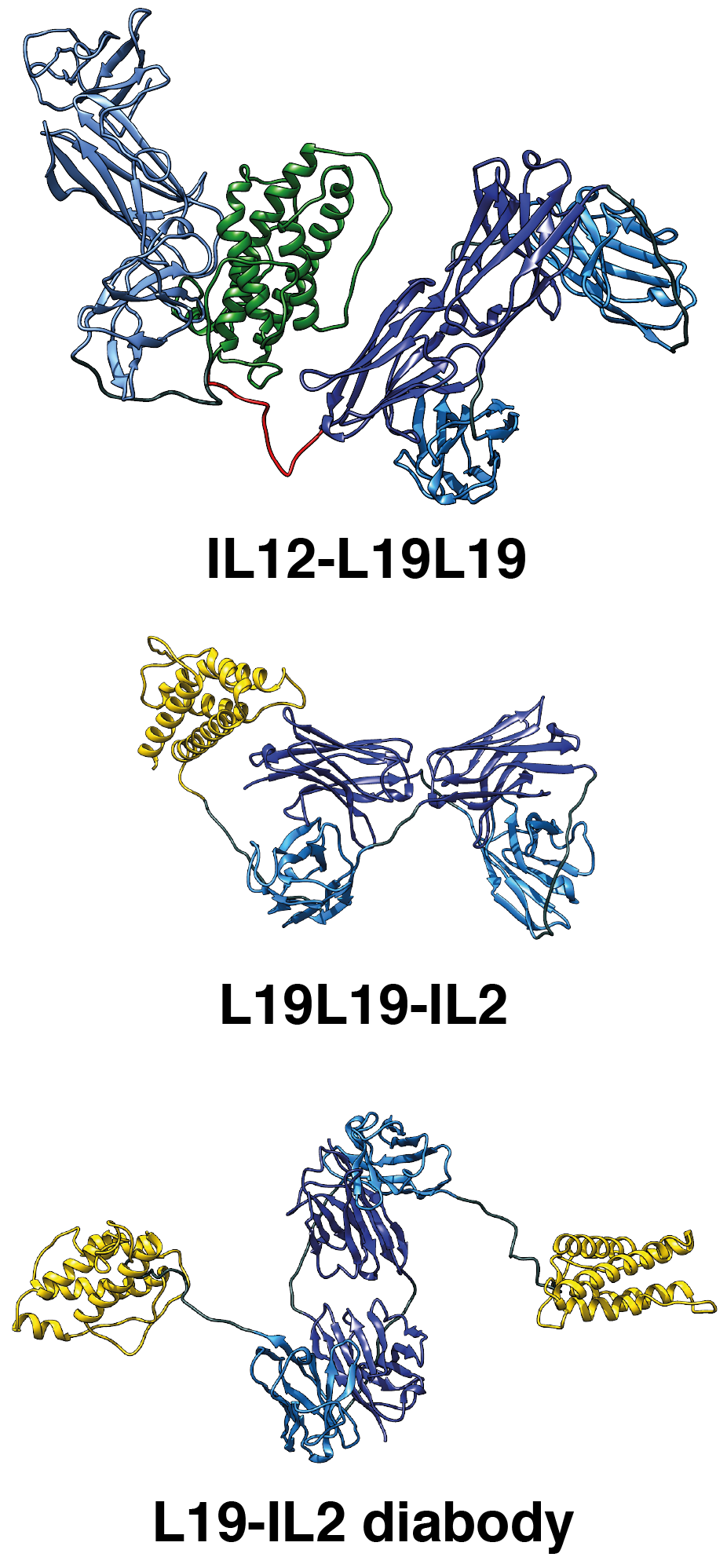
|
Cartoon representation of the molecular models of L19-IL2, L19L19-IL2 and IL12-L19L19 immunocytokines based on SEC-SAXS data. |
PDB Files:
SASBDB Files:
Raw Data in Repository:
Publications:
Inference of molecular structure for characterization and improvement of clinical grade immunocytokines.
Journal of Structural Biology, 107696. (2021) *Equal Contribution - **Shared Corresponding Authors
PubMed
Thanks to a collaboration with Prof. I Frébort and P. Galuszka ( University of Holomuc, Czech Republic), we have been involved in structural characterization of a recently discovered class of proteins, named "Lonely Guy" or LOG. These are key enzymes of cytokinin biosynthesis, converting inactive cytokinin nucleotides into biologically active free-bases. We have determined the three-dimensional structure of a fungal LOG protein from C. purpurea in ligand-free and ligand-bound states. Our structural and biochemical analysis provided insights into the mechanism of substrate recognition in these enzymes and accurate sequence signature to identify Rossmann fold-containing LOG-like phosphoribonucleotide-processing enzymes, to distinguish them from mis- annotated lysine decarboxylases.
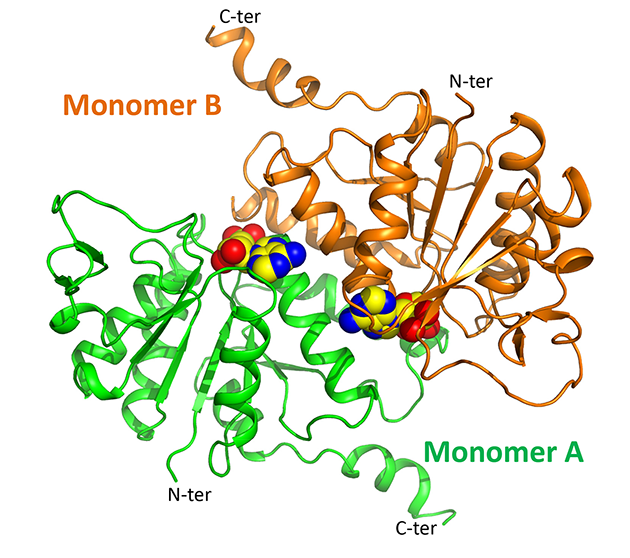
|
Overview of the three-dimensional structure of cpLOG, rendered as cartoon. The reaction product phosphoribose, identified in the catalytic pocket of the enzyme, is shown as spheres. |
PDB Files:
Raw Data in Repository:
Publications:
The three-dimensional Structure of Lonely Guy from Claviceps purpurea provides insights into the phosphoribohydrolase function of Rossmann fold-containing lysine decarboxylase-like proteins.
Proteins, 83, 1539-1546. (2015) *Shared Corresponding Authors -
PubMed
Targeting Burkholderia cenocepacia quorum sensing virulence to fight cystic fibrosis
Burkholderia cenocepacia is a rare opportunistic pathogen, considered a major concern among respiratory tract infections in cystic fibrosis patients. This pathogen is particularly difficult to treat because of its high level of resistance to the clinically relevant antimicrobial agents. In B. cenocepacia, the quorum sensing cell-cell communication system is involved in many processes critical for bacterial virulence, such as biofilm formation, or protease and siderophore production. Targeting the enzymes involved in this process represents a promising therapeutic approach. This research is performed in collaboration with the Molecular Microbiology Group of our Department, led by Prof. Giovanna Riccardi.
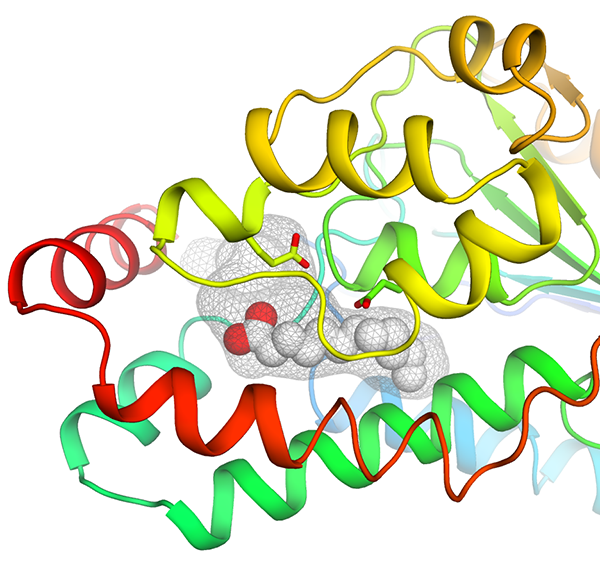
|
DfsA - The bifunctional crotonase DfsA is a key player in B. cenocepacia quorum sensing pathway. In this work, we presented the three-dimensional structure of the enzyme, fortuitously found in complex with the non- physiological reaction product lauric acid. The biochemical characterization of the enzyme revealed that, differently from the physiological product BDSF ( Burkholderia Diffusible Signaling Factor, cis-2-dodecenoic acid ), lauric acid is slowly released behaving as an inhibitory end product. Collectively, our data illustrate how this enzyme is capable to accommodate long substrate aliphatic side chains, such as its physiological substrate BDSF, without the need for previously suggested conformational changes. These results provide valuable structural templates for future structure-based drug development to target Burkholderia virulence, a trending approach in the fight against bacterial infections affecting cystic fibrosis patients. The figure shows a cartoon representation of the DfsA three- dimensional structure, colored from N-terminus (blue) to C-terminus (red), highlighting the deep hydrophobic cavity (grey mesh). The non-physiological reaction product lauric acid, identified near the catalytic pocket of the enzyme, is shown as spheres. |
|
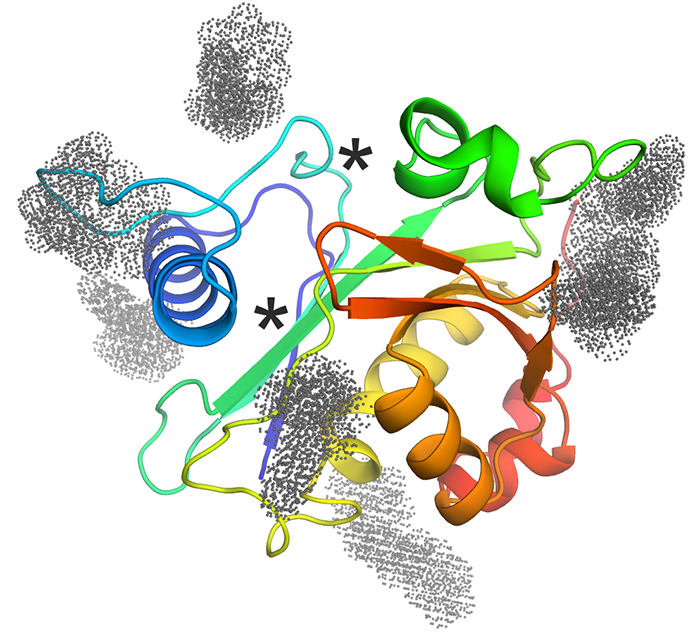
CepI - Our collaborators identified effective non-competitive inhibitors of B. cenocepacia acyl homoserine lactone synthase CepI. Unfortunately, this enzyme refused to crystallize, neither in absence nor in presence of these newly identified inhibitors. Thus, we performed homology modeling and in silico docking of the candidate inhibitors. Using in vitro mutagenesis and biochemical assays, we have validated the docking results to understand their mechanism of action of these powerful candidate drugs. The figure shows a cartoon representation of the CepI homology model, colored from N-terminus (blue) to C-terminus (red). The known substrate binding sites are shown with asterisks. Optimized inhibitor binding sites identified by molecular docking are shown with colored dots. |
PDB Files:
Files in Repository:
Publications:
Frontiers in Pharmacology, 8, 836. (2018) *Equal Contribution
Biochemistry, 55, 3241-3250. (2016) *Equal Contribution - **Shared Corresponding Authors -
PubMed
Scientific Reports, 6, 32487 (2016) *Equal Contribution - PubMed
PLoS One, 11, pp. e0167350. (2016) - PubMed
Completed Grants related to this research:
