Research Topics:
Characterizing Molecular Recognition and Regulation Events at Neuromuscular Junctions
Signaling between nerves and muscle cells requires the presence of stable synapses, called neuromuscular junctions (NMJ). During NMJ development, clustering of acetylcholine receptors (AChRs) on the muscle cell membrane is crucial for the formation of the synaptic connections. Despite the large number of studies, very little is known about the various steps required to form a functional neuromuscular synapse and how this process leads to the transfer of messages from the neurons to the muscles. A key process in AChRs clustering is the interaction between the large proteoglycan Agrin to its receptor, a macromolecular complex located on the muscle cell membrane constituted by the co-receptor low-density lipoprotein receptor-related protein 4 (LRP4) and muscle-specific receptor tyrosine kinase (MuSK). Direct binding of Agrin to LRP4 causes conformational changes in the co-receptor resulting in MuSK auto-activation and downstream signalling cascades. Ultimately, Agrin- mediated activation of its receptor triggers RapSyn-mediated clustering of AChRs on the surface of the muscle cell. This process is regulated by proteolytic cleavage of Agrin by a synapse-specific protease called Neurotrypsin. Together, these proteins have strong implications in congenital and autoimmune neuromuscular diseases, including highly invalidating myasthenia gravis (affecting to date 15-20 people per populations of 100,000). We use several experimental techniques to investigate the molecular structures and functions of synaptic ligands and receptors to elucidate their roles in the processes that lead to formation and stabilization of neuromuscular junctions. We combine the knowledge about the functions of these proteins with in-depth biochemical and biophysical characterizations of their mutual interactions.
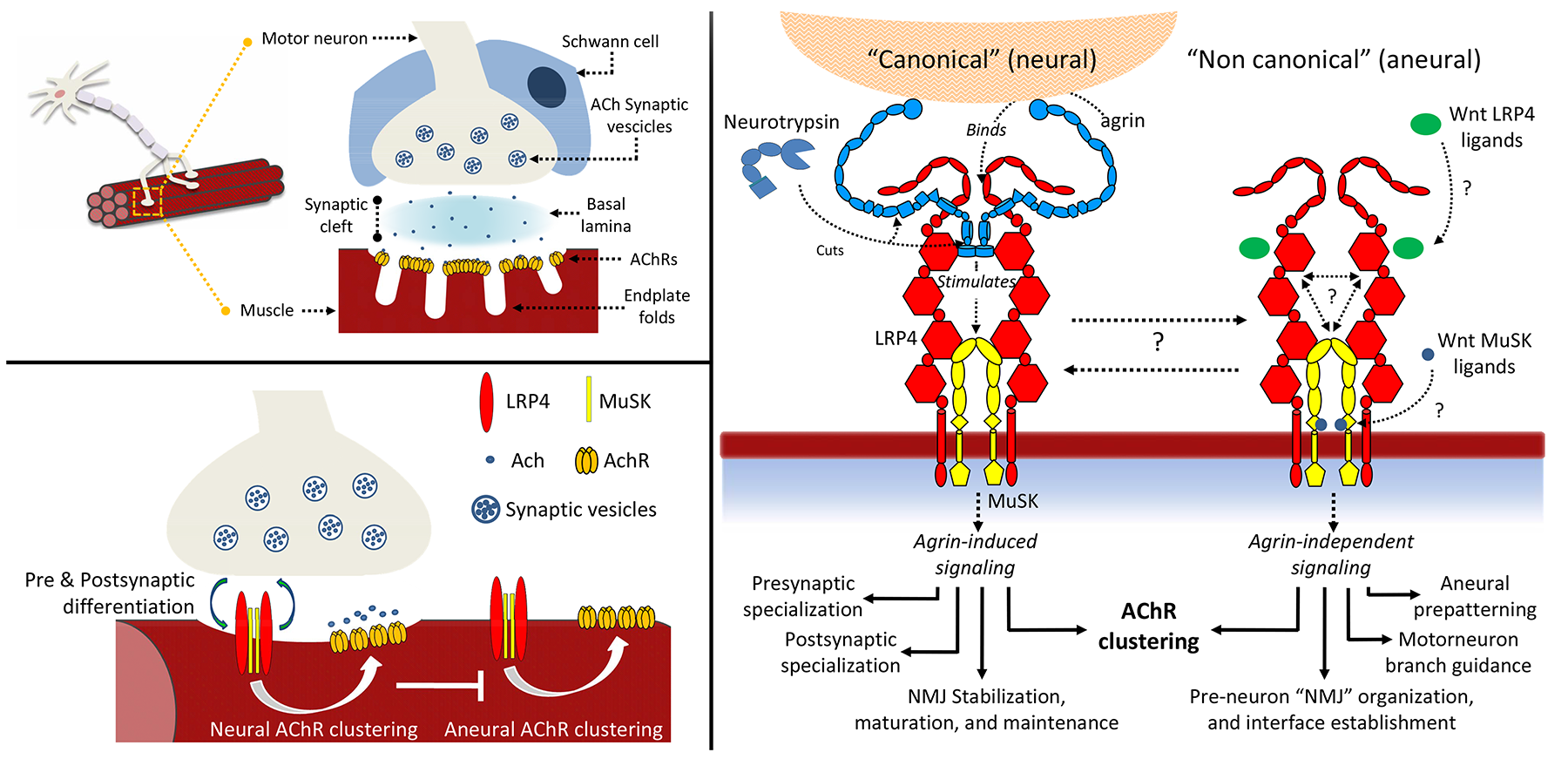
The figure shows an overview of the complexity underlying neuromuscular junction formation and their extracellular organizers: the agrin receptor composed of LRP4:MuSK and its associated effectors.
The ultimate long-term goal of our research is to provide an accurate "molecular blueprint" of the synapse environment and to transpose the observations at the cellular level. Gathering knowledge about the mechanisms of neuromuscular junctions homeostasis will contribute important knowledge for future design of compounds to potentially counter-act the effects of important neuromuscular dysfunctions (including myasthenia gravis), with important consequences on the quality of life of the patients affected by neuromuscular diseases.
|
Neurotrypsin - Neurotrypsin (NT) is a multi-domain serine protease of the nervous system with only one known substrate: the large proteoglycan Agrin. NT has seen to be involved in the maintenance/turnover of neuromuscular junctions and in processes of synaptic plasticity in the central nervous system. Roles which have been tied to its enzymatic activity, localized in the C-terminal serine-protease (SP) domain. However the purpose of NT's remaining 3-4 scavenger receptor cysteine-rich ( SRCR) domains is still unclear. We have determined the crystal structure of the third SRCR domain of murine NT (mmNT-SRCR3), immediately preceding the SP domain and performed a comparative structural analysis using homologous SRCR structures. Our data and the elevated degree of structural conservation with homologous domains highlight possible functional roles for NT SRCRs. Computational and experimental analyses suggest the identification of a putative binding region for Ca2+ ions, known to regulate NT enzymatic activity. Furthermore, sequence and structure comparisons allow to single out regions of interest that, in future studies, might be implicated in Agrin recognition/ binding or in interactions with as of yet undiscovered NT partners. |
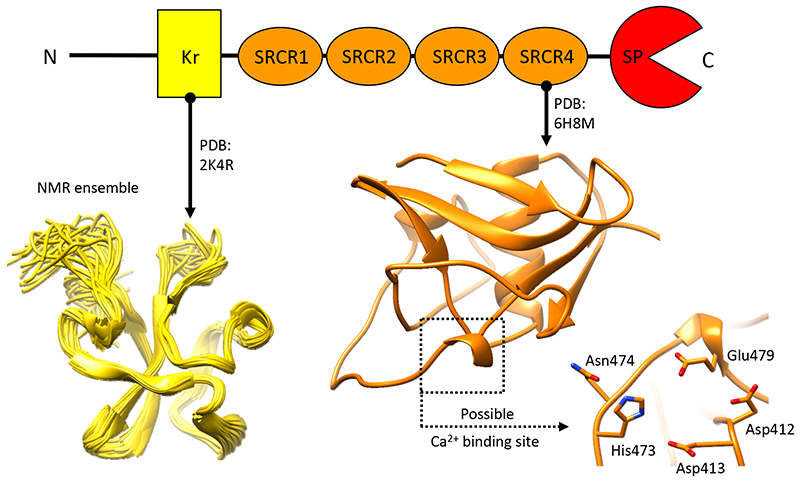
The figure shows a cartoon representation of the three-dimensional structure of the third scavenger receptor cysteine-rich domain of mouse Neurotrypsin (NT-CRD3). |
PDB Files:
SASBDB Files:
Files in Repository:
Publications:
Deconstruction of Neurotrypsin Reveals a Multi-factorially Regulated Activity Affecting Myotube Formation and Neuronal Excitability.
Molecular Neurobiology, 59, 7466-7485 (2022) *Corresponding Authors
- PubMed
Dissecting the extracellular complexity of neuromuscular junction organizers.
Frontiers in Molecular Biosciences, 6, 156. (2020) *Equal Contribution
- PubMed
Structural characterization of the third scavenger receptor cysteine-rich domain of murine Neurotrypsin.
Protein Science, 28, 746-755. (2019)
- PubMed
Active Grants related to this research:
Completed Grants related to this research:
